Exactech is a global medical technology company that empowers orthopaedic surgeons with innovative implants, surgical instruments and the Active Intelligence® (AI) ecosystem of smart technologies to give patients exactly what they need to regain mobility. The company successfully launched its Vantage® Total Ankle System, yet the team were exploring opportunities to help surgeons more. One possibility was to enable surgeons to personalize their procedures with patient-specific surgical guides that pair with Exactech’s implants.
Additive manufacturing (AM) demonstrated its ability to personalize craniomaxillofacial procedures, and Exactech felt this would transfer to ankle replacements. Exactech selected 3D Systems to develop a new patient-specific instrumentation (PSI) solution comprising both surgical planning and patient-specific surgical guides. By pairing the PSI offering with its implants for total ankle replacement procedures, Exactech reaffirmed its role as a leader in the foot and ankle market.
“This was an opportunity to make big gains in the lives of patients and surgeons. We wanted to develop a PSI solution that would take reproducibility and accuracy to new levels, while measurably decreasing time in the OR. 3D Systems was the right partner for us.”
- Devan Carter, Director of Marketing, Foot & Ankle, Exactech
The Challenge
How Can We Drive Better Results for Surgeons and Their Patients?
Total ankle replacement has become progressively more predictable and effective over the years. Yet, surgeons and patients still face a number of risks, including subsidence, implant loosening, bone cysts and impingement, which all can lead to pain and revision surgeries. Addressing these risks—and driving measurable efficiency gains in the OR—became key goals for the joint development effort between Exactech and 3D Systems. “We started by asking ourselves: how can we better drive results for surgeons and their patients? And we began innovating there,” Carter said.
Vantage Coupled Curve Talus
Vantage 3D Tibia
The Solution
Patient-Specific Instrumentation and Pre-Surgical Planning
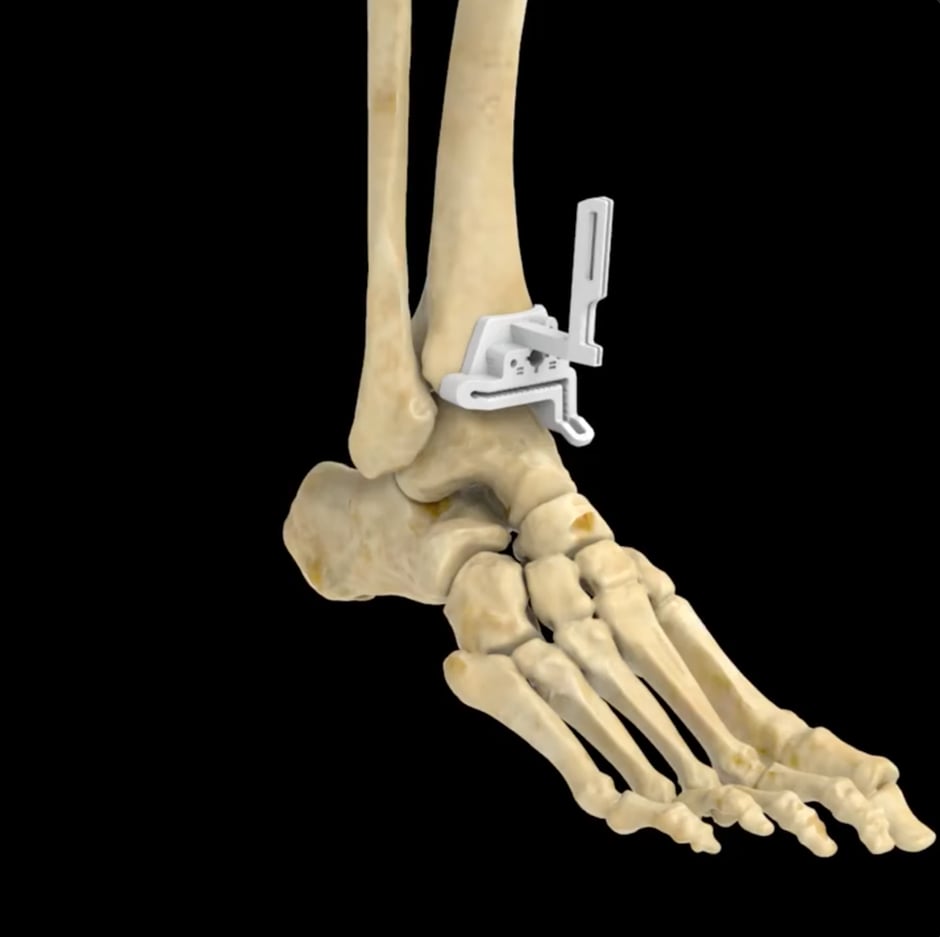
Together, Exactech and 3D Systems developed the Vantage® Ankle PSI, which includes pre-surgical planning and a patient-specific 3D-printed instrument set that is used to guide resections in the tibia and talus. Through and intuitive planning portal, surgeons can personalize treatment plans with the help of 3D Systems dedicated team of engineers, who tailor each guide to specific patient anatomies leveraging the company’s FDA-cleared VSP® surgical planning workflows. Then, 3D Systems manufactures the PSIs using its SLS Duraform® ProX™ PA material and delivers them to the surgeon along with the surgical plan.
Surgeons have been highly receptive to the company’s complete ankle solution—now with Vantage® Ankle, Vantage® 3D and Vantage® 3D+ patient-specific guides—because they are able to create highly detailed plans before heading into the OR. Meanwhile, detailed 3D anatomical models alongside the personalized guides allow surgeons to translate the surgical plan in the OR.
Response to the solution has been overwhelmingly positive, according to Carter. “About 50 percent of the cases we support involve PSI and the numbers are growing. Once a surgeon uses the solution and they see how it empowers them—and how it saves time in the OR—they tend not to go back.”
OR Efficiency Gains and Improved Patient Recovery
The Vantage Ankle PSI has the potential to increase OR efficiency by allowing surgeons to reduce the number of steps required to prepare the anatomy through the use of a patient-matched 3D-printed instrument set.
With standard instrumentation, surgeons are required to manually adjust the alignment of their cuts with an external jig. Making necessary adjustments to those placements requires a large number of x-rays. With Vantage Ankle PSI, surgeons take the guide, place it in the right spot, find the landmarks, check the original images, and then proceed.
One of the key PSI features that makes the device unique is its large footprint that accurately seats the guide on the bone anatomy. Other key innovations include improved visibility to alignment and corrugated cutting slots that aid surgical irrigation. Soft tissue offsets also help preserve the periosteum—the outer fibrous layer of the bone, which helps aid in its healing and recovery.
View Darylyn’s story to see how the solution helped her surgeon restore her mobility.
Market Reception: Nearly 50% of Ankle Replacements and Growing
Market adoption of the Vantage Ankle PSI has been strong—about half of Exactech’s ankle engagements now include this solution. A key reason for strong adoption is that surgeons are finding that it not only reduces uncertainty during the procedure, but the simplified procedure process also saves significant OR time per procedure.
Foot and ankle specialists tend to be among the most progressive surgeons, especially in the U.S. One reason why these early adopters have gravitated to Vantage Ankle PSI is that reports can be set to reflect their preferences. Exactech’s interactive planning portal also allows them to experiment with sizing and cuts in the days leading up to the surgery, so they are comfortable with their plan heading into the OR. Even with these self-guided tools, most take advantage of the ability to speak with 3D Systems engineers during the planning process.

